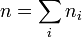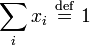In chemistry, a solution is a homogeneous mixture composed of two or more substances. In such a mixture, a solute is dissolved in another substance, known as a solvent.
Types of solutions
Usually, the substance present in a greater amount is considered as the solvent. Solvents can be gases, liquids, or solids. The solution that forms has the same physical state as the solvent.Gas
If the solvent is a gas, only gases can be dissolved. An example for a gaseous solution is air (oxygen and other gases dissolved in nitrogen). Since interactions between molecules play almost no role, dilute gases form rather trivial solutions. In part of the literature, they are not even classified as solutions, but addressed as mixtures.Liquid
If the solvent is a liquid, gases, liquids, and solids can be dissolved. Examples are:- Gas in liquid:
- Oxygen in water.
- Carbon dioxide in water is a less simple example, because the solution is accompanied by a chemical reaction (formation of ions). Note also that the visible bubbles in carbonated water are not the dissolved gas, but only an effervescence; the dissolved gas itself is not visible since it is dissolved on a molecular level.
- Liquid in liquid:
- Alcoholic beverages are basically solutions of ethanol in water.
- Petroleum is a solution of various hydrocarbons.
- Solid in liquid:
- Sucrose (table sugar) in water
- Sodium chloride or any other salt in water forms an electrolyte: When dissolving, salt dissociates into ions.
Body fluids are examples for complex liquid solutions, containing many different solutes. They are electrolytes since they contain solute ions (e.g. potassium and sodium). Furthermore, they contain solute molecules like sugar and urea. Oxygen and carbon dioxide are also essential components of blood chemistry, where significant changes in their concentrations can be a sign of illness or injury.
Solid
If the solvent is a solid, gases, liquids, and solids can be dissolved.- Gas in solid:
- Hydrogen dissolves rather well in metals, especially in palladium; this is studied as a means of hydrogen storage.
- Liquid in solid:
- mercury in gold, forming an amalgam
- Hexane in paraffin wax
- Solid in solid
- Steel, basically a solution of carbon atoms in a crystalline matrix of iron atoms.
- Alloys like bronze and many others.
- Polymers containing plasticizers.
Solubility
Main article: Solubility
Main article: Solvation
The ability of one compound to dissolve in another compound is called solubility. When a liquid is able to completely dissolve in another liquid the two liquids are miscible. Two substances that can never mix to form a solution are called immiscible.All solutions have a positive entropy of mixing. The interactions between different molecules or ions may be energetically favored or not. If interactions are unfavorable, then the free energy decreases with increasing solute concentration. At some point the energy loss outweighs the entropy gain, and no more solute particles can be dissolved; the solution is said to be saturated. However, the point at which a solution can become saturated can change significantly with different environmental factors, such as temperature, pressure, and contamination. For some solute-solvent combinations a supersaturated solution can be prepared by raising the solubility (for example by increasing the temperature) to dissolve more solute, and then lowering it (for example by cooling).
Usually, the greater the temperature of the solvent, the more of a given solid solute it can dissolve. However, most gases and some compounds exhibit solubilities that decrease with increased temperature. Such behavior is a result of an exothermic enthalpy of solution. Some surfactants exhibit this behaviour. The solubility of liquids in liquids is generally less temperature-sensitive than that of solids or gases.
Properties
The physical properties of compounds such as melting point and boiling point change when other compounds are added. Together they are called colligative properties. There are several ways to quantify the amount of one compound dissolved in the other compounds collectively called concentration. Examples include molarity, mole fraction, and parts per million (ppm).
The properties of ideal solutions can be calculated by the linear combination of the properties of its components. If both solute and solvent exist in equal quantities (such as in a 50% ethanol, 50% water solution), the concepts of "solute" and "solvent" become less relevant, but the substance that is more often used as a solvent is normally designated as the solvent (in this example, water).
Liquid solutions
In principle, all types of liquids can behave as solvents: liquid noble gases, molten metals, molten salts, molten covalent networks, molecular liquids. In the practice of chemistry and biochemistry, most solvents are molecular liquids. They can be classified into polar and non-polar, according to whether or not molecules possess a permanent electric dipole moment. Another distinction is whether or not molecules are able to form hydrogen bonds (protic and aprotic solvents). Water, the most commonly used solvent, is both polar and sustaining hydrogen bonds.Salts dissolve in polar solvents, forming positive and negative ions that are attracted to the positive and negative ends of the solvent molecule, respectively. If the solvent is water, hydration occurs when the charged solute ions become surrounded by water molecules. A standard example is aqueous saltwater. Such solutions are called electrolytes.
For non-ionic solutes, the general rule is: like dissolves like.
Polar solutes dissolve in polar solvents, forming polar bonds or hydrogen bonds. As an example, all alcoholic beverages are aqueous solutions of ethanol. On the other hand, non-polar solutes dissolve better in non-polar solutes. Examples are various hydrocarbons like oil and grease that easily mix with each other, while being incompatible with water.
Examples for the immiscibility of oil and water are Italian salad dressing and petroleum, leaking from a damaged tanker, that does not dissolve in the ocean water but rather floats on the surface.
Concentration of Solutions
In chemistry, concentration is the measure of how much of a given substance there is mixed with another substance. This can apply to any sort of chemical mixture, but most frequently the concept is limited to homogeneous solutions, where it refers to the amount of solute in the solvent.To concentrate a solution, one must add more solute, or reduce the amount of solvent (for instance, by selective evaporation). By contrast, to dilute a solution, one must add more solvent, or reduce the amount of solute.
Unless two substances are fully miscible there exists a concentration at which no further solute will dissolve in a solution. At this point, the solution is said to be saturated. If additional solute is added to a saturated solution, it will not dissolve (except in certain circumstances, when supersaturation may occur). Instead, phase separation will occur, leading to either coexisting phases or a suspension. The point of saturation depends on many variables such as ambient temperature and the precise chemical nature of the solvent and solute.
Analytical concentration includes all the forms of that substance in the solution.
Qualitative description
Often in informal, non-technical language, concentration is described in a qualitative way, through the use of adjectives such as "dilute" for solutions of relatively low concentration and of others like "concentrated" for solutions of relatively high concentration. Those terms relate the amount of a substance in a mixture to the observable intensity of effects or properties caused by that substance. For example, a practical rule is that the more concentrated a chromatic solution is, the more intensely colored it is (usually).
Quantitative notation
For scientific or technical applications, a qualitative account of concentration is almost never sufficient; therefore quantitative measures are needed to describe concentration. There are a number of different ways to quantitatively express concentration; the most common are listed below. They are based on mass, volume, or both. Depending on what they are based on it is not always trivial to convert one measure to the other, because knowledge of the density might be needed to do so. At times this information may not be available, particularly if the temperature varies.Mass versus volume
Units of concentration — particularly the most popular one, molarity — require knowledge of a substance's volume, which unlike mass is variable depending on ambient temperature and pressure. In fact (partial) molar volume can even be a function of concentration itself. This is why volumes are not necessarily completely additive when two liquids are added and mixed. Volume-based measures for concentration are therefore not to be recommended for non-dilute solutions or problems where relatively large differences in temperature are encountered (e.g. for phase diagrams).Unless otherwise stated, all the following measurements of volume are assumed to be at a standard state temperature and pressure (for example 0 degrees Celsius at 1 atmosphere or 101.325 kPa). The measurement of mass does not require such restrictions.
Mass can be determined at a precision of < 0.2 mg on a routine basis with an analytical balance and more precise instruments exist. Both solids and liquids are easily quantified by weighing.
The volume of a liquid is usually determined by calibrated glassware such as burettes and volumetric flasks. For very small volumes precision syringes are available. The use of graduated beakers and cylinders is not recommended as their indication of volume is mostly for decorative rather than quantitative purposes. The volume of solids, particularly of powders, is often difficult to measure, which is why mass is the more usual measure. For gases the opposite is true: the volume of a gas can be measured in a gas burette, if care is taken to control the pressure, but the mass is not easy to measure due to buoyancy effects.
Molarity
Molarity (in units of mol/L, molar, or M) or molar concentration denotes the number of moles of a given substance per liter of solution. A capital letter M is used to abbreviate units of mol/L. For instance:Following the SI system of units, the National Institute of Standards and Technology, the United States authority on measurement, considers the term molarity and the unit symbol M to be obsolete, and suggests instead the amount-of-substance concentration (c) with the units mol/m3 or other units used alongside the SI such as mol/L[1]. This recommendation has not been universally implemented in academia or chemistry research yet.
Preparation of a solution of known molarity involves adding an accurately weighed amount of solute to a volumetric flask, adding some solvent to dissolve it, then adding more solvent to fill to the volume mark.
When discussing molarity of minute concentrations, such as in pharmacological research, molarity is expressed in units of millimolar (mmol/L, mM, 1 thousandth of a molar), micromolar (μmol/L, μM, 1 millionth of a molar) or nanomolar (nmol/L, nM, 1 billionth of a molar).
Although molarity is by far the most commonly used measure of concentration, particularly for dilute aqueous solutions, it does suffer from a number of disadvantages. Masses can be determined with great precision as balances are often very precise. Determining volume is often not as precise. In addition, due to a thermal expansion, molarity of a solution changes with temperature without adding or removing any mass.[2] For non-dilute solutions another problem is the molar volume of a substance is itself a function of concentration so volume is not strictly additive.
Molality
Molality (mol/kg, molal, or m) denotes the number of moles of solute per kilogram of solvent (not solution). For instance: adding 1.0 mole of solute to 2.0 kilograms of solvent constitutes a solution with a molality of 0.50 mol/kg. Such a solution may be described as "0.50 molal". The term molal solution is used as a shorthand for a "one molal solution", i.e. a solution which contains one mole of the solute per 1000 grams of the solvent.Following the SI system of units, the National Institute of Standards and Technology, the United States authority on measurement, considers the unit symbol m to be obsolete, and suggests instead the term 'molality of substance B' (mB) with units mol/kg or a related unit of the SI[3]. This recommendation has not been universally implemented in academia yet.
Note: molality is sometimes represented by the symbol (m), while molarity by the symbol (M). The two symbols are not meant to be confused, and should not be used as symbols for units. The SI unit for molality is mol/kg. (The unit m means meter.)
Like other mass-based measures, the determination of molality only requires a good scale, because masses of both solvent and solute can be obtained by weighing, and molality is independent of physical conditions like temperature and pressure, providing advantages over molarity.
In a dilute aqueous solution near room temperature and standard atmospheric pressure, molarity and molality will be very similar in value. This is because 1 kg of water roughly corresponds to a volume of 1 L at these conditions, and because the solution is dilute, the addition of the solute makes a negligible impact on the volume of the solution.
However, in all other conditions, this is usually not the case.
Mole fraction
Mole fraction x (also, and more correctly, known as the amount fraction) is a way of expressing the composition of a mixture. The mole fraction of each component i is defined as its amount of substance ni divided by the total amount of substance in the system, nThe same value for the mole fraction ratio is obtained using the number of molecules of i, Ni, and the total number of molecules of all kinds, N, since
Notes and qualifications
Mole fractions are dimensionless numbers. Other ways of representing concentrations, e.g., molarity and molality, yield dimensional quantities (per litre, per kilogram, etc.). When chemical formulas seem to be taking the logarithms of dimensional quantities, there is an implied ratio, and such expressions can always be rearranged so that the arguments of the logarithms are dimensionless numbers, as they must be.Mole fractions are one way of representing the concentrations of the various chemical species. They are an ideal-mixture approximation to the effect of concentration on the equilibrium or rate of a reaction. In practice (except for very dilute solutions or for gasses at atmospheric pressure), all measures of concentration must be multiplied by correction factors called activity coefficients in order to yield accurate results.
The mole fraction is sometimes denoted by the lower case Greek letter χ (chi) instead of a Roman x. For mixtures of gases, it is more usual to use the letter y. The mole fraction of a substance in a reaction is also equal to the partial pressure of that substance.
Mass percentage (fraction)
Mass percentage denotes the mass of a substance in a mixture as a percentage of the mass of the entire mixture. (Mass fraction xm can be used instead of mass percentage by dividing mass percentage to 100.) Commercial concentrated aqueous reagents such as acids and bases are often labeled in concentrations of weight percentage with the specific gravity also listed. In older texts and references this is sometimes referred to as weight-weight percentage (abbreviated as w/w% [4] or wt%). In water pollution chemistry, a common term of measuring total mass percentage of dissolved solids in an aqueous medium is total dissolved solids.For instance: if a bottle contains 40 grams of ethanol and 60 grams of water, then it contains 40% ethanol by mass or 0.4 mass fraction ethanol. Note that the total weight of the solution will be 100 grams, but the total volume of the solution will be more than 100 milliliters because ethanol is less dense than water.
Mass-volume percentage
Mass-volume percentage, (sometimes referred to as weight-volume percentage or percent weight per volume and often abbreviated as % m/v or % w/v) describes the mass of the solute in g per 100 mL of the resulting solution. Mass-volume percentage is often used for solutions made from a solid solute dissolved in a liquid. For example, a 40% w/v sugar solution contains 40 g of sugar per 100 mL of resulting solution.Volume-volume percentage
Main article: Volume percent
Volume-volume percentage (sometimes referred to as percent volume per volume and abbreviated as % v/v) describes the volume of the solute in mL per 100 mL of the resulting solution. This is most useful when a liquid - liquid solution is being prepared, although it is used for mixtures of gases as well. For example, a 40% v/v ethanol solution contains 40 mL ethanol per 100 mL total volume. The percentages are only additive in the case of mixtures of ideal gases.Normality
Normality highlights the chemical nature of salts: in solution, salts dissociate into distinct reactive species (ions such as H+, Fe3+, or Cl-). Normality accounts for any discrepancy between the concentrations of the various ionic species in a solution. For example, in a salt such as MgCl2, there are two moles of Cl- for every mole of Mg2+, so the concentration of Cl- is said to be 2 N (read: "two normal"). Further examples are given below. It may also refer to the concentration of a solute in any solution. The normality of a solution is the number of gram equivalent weight of a solute per litre of its solution. For example hydrochloric acid(HCl). One litre of aqueous solution of HCl acid contains 36.5 grams HCl. It is called 1N (one normal)solution of HCl. It is given by the following formula:Definition
A normal is one gram equivalent of a solute per liter of solution. The definition of a gram equivalent varies depending on the type of chemical reaction that is discussed - it can refer to acids, bases, redox species, and ions that will precipitate.Usage
It is critical to note that normality measures a single ion which takes part in an overall solute. For example, one could determine the normality of hydroxide or sodium in an aqueous solution of sodium hydroxide, but the normality of sodium hydroxide itself has no meaning. Nevertheless it is often used to describe solutions of acids or bases, in those cases it is implied that the normality refers to the H+ or OH− ion. For example, 2 Normal sulfuric acid (H2SO4), means that the normality of H+ ions is 2, or that the molarity of the sulfuric acid is 1. Similarly for 1 molar H3PO4 the normality is 3 as it contains three moles of H+ ions for every mole of PO43- .Specific cases
As ions in solution can react through different pathways, there are three common definitions for normality as a measure of reactive species in solution:- In acid-base chemistry, normality is used to express the concentration of protons or hydroxide ions in the solution. Here, the normality differs from the molarity by an integer value - each solute can produce n equivalents of reactive species when dissolved. For example: 1 M aqueous Ca(OH)2 is 2 N (normal) in hydroxide.
- In redox reactions, normality measures the quantity of oxidizing or reducing agent that can accept or furnish one mole of electrons. Here, the normality scales from the molarity, most commonly, by a fractional value. Calculating the normality of redox species in solution can be challenging.
- In precipitation reactions, normality measures the concentration of ions which will precipitate in a given reaction. Here, the normality scales from the molarity again by an integer value.
Practical uses
The measure of normality is extremely useful for titrations - given two species that are known to react with a known ratio, one simply needs to scale the volumes of solutions with known normalities to get a complete reaction with the following equation:NaVa=NbVb
However, normality cannot reliably represent an unambiguous measure of the concentration of a solution. Since the measure of normality depends on the reaction that the solute participates in, the same concentration of solute can possess two different normalities for two different reactions. For example, Mg2+ is 2 N with respect to a Cl- ion, but it is only 1 N with respect to an O2- ion.
Accordingly, normality is no longer used to represent the concentration of a solution as such. Instead, a solution should be labeled according to its molarity, and it is then possible to calculate the normality for a particular titration using the equation above. NIST has also stipulated that this unit is obsolete and recommends discontinuing its use.
Equivalents
Expression of concentration in equivalents per liter (or more commonly, milliequivalents per liter) is based on the same principle as normality. A normal solution is one equivalent per liter of solution (Eq/L). The use of equivalents and milliequivalents as a means of expressing concentration is losing favor, but medical reporting of serum concentrations in mEq/L still occurs.Formal
The formal (F) is yet another measure of concentration similar to molarity. Formal concentrations are sometimes used when solving chemical equilibrium problems. It is calculated based on the formula weights of chemicals per liter of solution. The difference between formal and molar concentrations is that the formal concentration indicates moles of the original chemical formula in solution, without regard for the species that actually exist in solution. Molar concentration, on the other hand, is the concentration of species in solution.For example: if one dissolves sodium carbonate (Na2CO3) in a liter of water, the compound dissociates into the Na+ and CO32- ions. Some of the CO32- reacts with the water to form HCO3- and H2CO3. If the pH of the solution is low, there is practically no Na2CO3 left in the solution. So, although we have added 1 mol of Na2CO3 to the solution, it does not contain 1 M of that substance. (Rather, it contains a molarity based on the other constituents of the solution.) However, it was once said that such solutions contain 1 F of Na2CO3.
"Parts-per" notation
Main article: Parts-per notation
The parts-per notation is used in some areas of science and engineering because it does not require conversion from weights or volumes to more chemically relevant units such as normality or molarity. It describes the amount of one substance in another, and is thus related to the mass fraction. It is the ratio of the amount of the substance of interest to the amount of that substance plus the amount of the substance it is in.- Parts per hundred (denoted by '%' [the per cent symbol], and very rarely 'pph') - denotes the amount of a given substance in a total amount of 100 regardless of the units of measure as long as they are the same. e.g. 1 gram per 100 gram. 1 part in 102.
- Parts per thousand (denoted by '‰' [the per mille symbol], and occasionally 'ppt', but this usage can be confusing because it more often denotes parts per trillion) denotes the amount of a given substance in a total amount of 1000 regardless of the units of measure as long as they are the same. e.g. 1 milligram per gram, or 1 gram per kilogram. 1 part in 103.
- Parts per million ('ppm') denotes the amount of a given substance in a total amount of 1,000,000 regardless of the units of measure used as long as they are the same. e.g. 1 milligram per kilogram. 1 part in 106.
- Parts per billion ('ppb') denotes the amount of a given substance in a total amount of 1,000,000,000 regardless of the units of measure as long as they are the same. e.g. 1 milligram per tonne. 1 part in 109.
- Parts per trillion ('ppt') denotes the amount of a given substance in a total amount of 1,000,000,000,000 regardless of the units of measure as long as they are the same. e.g. 1 milligram per kilotonne. 1 part in 1012.
- Parts per quadrillion ('ppq') denotes the amount of a given substance in a total amount of 1,000,000,000,000,000 regardless of the units of measure as long as they are the same. e.g. 1 milligram per megatonne. 1 part in 1015.
Notes for clarity
The notation is used for convenience and the units of measure must be denoted for clarity though this is frequently not the case even in technical publications.In atmospheric chemistry and in air pollution regulations, the parts per notation is commonly expressed with a v following, such as ppmv, to indicate parts per million by volume. This works fine for gas concentrations (e.g., ppmv of carbon dioxide in the ambient air) but, for concentrations of non-gaseous substances such as aerosols, cloud droplets, and particulate matter in the ambient air, the concentrations are commonly expressed as μg/m3 or mg/m3 (e.g., μg or mg of particulates per cubic metre of ambient air). This expression eliminates the need to take into account the impact of temperature and pressure on the density and hence weight of the gas.
The usage is generally quite fixed inside most specific branches of science, leading some researchers to believe that their own usage (mass/mass, volume/volume or others) is the only correct one. This, in turn, leads them not to specify their usage in their research, and others may therefore misinterpret their results. For example, electrochemists often use volume/volume, while chemical engineers may use mass/mass as well as volume/volume. Many academic papers of otherwise excellent level fail to specify their usage of the part-per notation. The difference between expressing concentrations as mass/mass or volume/volume is quite significant when dealing with gases and it is very important to specify which is being used. It is quite simple, for example, to distinguish ppm by volume from ppm by mass or weight by using ppmv or ppmw.
Table of concentration measures
| Measurement | Notation | Generic formula | Typical units |
|---|---|---|---|
| atomic percentage (A) | at.% | 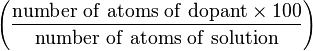 | % |
| atomic percentage (B) | at.% | 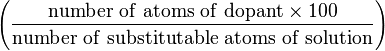 | % |
| Mass percentage | wt% |  | % |
| Mass-volume percentage | - | 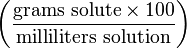 | % though strictly %g/mL |
| Volume-volume percentage | vol% | 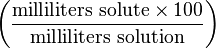 | % |
| Molarity | M | 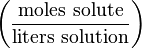 | mol/L (or M or mol/dm3) |
| Molinity | - | 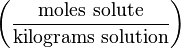 | mol/kg |
| Molality | m |  | mol/kg (or m**) |
| Molar fraction | Χ (chi) |  | (decimal) |
| Formal | F | 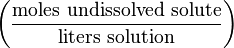 | mol/L (or F) |
| Normality | N |  | N |
| Parts per hundred | % (or pph) |  | dg/kg |
| Parts per thousand | ‰ (or ppt*) |  | g/kg |
| Parts per million | ppm |  | mg/kg |
| Parts per billion | ppb |  | µg/kg |
| Parts per trillion | ppt* |  | ng/kg |
| Parts per quadrillion | ppq |  | pg/kg |
** Obsolete unit symbols.