Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. Perhaps the most peculiar aspect of quantum mechanics is the quantization of observable quantities. This is distinguished from classical mechanics where the values can range continuously. They often describe specifically the energies of electrons in atoms, but other possibilities include angular momentum, spin etc. Since any quantum system can have one or more quantum numbers, it is a rigorous job to list all possible quantum numbers.
These are conventionally known as
Example: The quantum numbers used to refer to the outermost valence electron of the Carbon (C) atom, which is located in the 2p atomic orbital, are; n = 2 (group 2), l = 1 or 0, ml = 1, or 0, or −1, ms = −1/2 or 1/2.
Note that molecular orbitals require totally different quantum numbers, because the Hamiltonian and its symmetries are quite different.
Typical quantum numbers related to spacetime symmetries are spin (related to rotational symmetry), the parity, C-parity and T-parity (related to the Poincare symmetry of spacetime). Typical internal symmetries are lepton number and baryon number or the electric charge. (For a full list of quantum numbers of this kind see the article on flavour.)
It is worth mentioning here a minor but often confusing point. Most conserved quantum numbers are additive. Thus, in an elementary particle reaction, the sum of the quantum numbers should be the same before and after the reaction. However, some, usually called a parity, are multiplicative; ie, their product is conserved. All multiplicative quantum numbers belong to a symmetry (like parity) in which applying the symmetry transformation twice is equivalent to doing nothing. These are all examples of an abstract group called Z2.
For more detailed explanation ClickHere!
How many quantum numbers?
The question of how many quantum numbers are needed to describe any given system has no universal answer, although for each system one must find the answer for a full analysis of the system. The dynamics of any quantum system are described by a quantum Hamiltonian, H. There is one quantum number of the system corresponding to the energy, i.e., the eigenvalue of the Hamiltonian. There is also one quantum number for each operator O that commutes with the Hamiltonian (i.e. satisfies the relation OH = HO). These are all the quantum numbers that the system can have. Note that the operators O defining the quantum numbers should be independent of each other. Often there is more than one way to choose a set of independent operators. Consequently, in different situations different sets of quantum numbers may be used for the description of the same system.These are conventionally known as
- The principal quantum number (n = 1, 2, 3, 4 ...) denotes the eigenvalue of H with the J2 part removed[ambiguous]. This number therefore has a dependence only on the distance between the electron and the nucleus (ie, the radial coordinate, r). The average distance increases with n, and hence quantum states with different principal quantum numbers are said to belong to different shells.
- The azimuthal quantum number (l = 0, 1 ... n−1) (also known as the angular quantum number or orbital quantum number) gives the orbital angular momentum through the relation
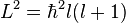 . In chemistry, this quantum number is very important, since it specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. In some contexts, l=0 is called an s orbital, l=1 a p orbital, l=2 a d orbital, and l=3 an f orbital.
. In chemistry, this quantum number is very important, since it specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. In some contexts, l=0 is called an s orbital, l=1 a p orbital, l=2 a d orbital, and l=3 an f orbital. - The magnetic quantum number (ml = −l, −l+1 ... 0 ... l−1, l) is the eigenvalue,
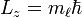 . This is the projection of the orbital angular momentum along a specified axis.
. This is the projection of the orbital angular momentum along a specified axis.
- The spin projection quantum number (ms = −1/2 or +1/2), is the intrinsic angular momentum of the electron. This is the projection of the spin s=1/2 along the specified axis.
| name | symbol | orbital meaning | range of values | value example |
|---|---|---|---|---|
| principal quantum number |  | shell |  |  |
| azimuthal quantum number (angular momentum) |  | subshell (s orbital is listed as 0, p orbital as 1 etc.) | 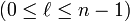 | for  : : |
| magnetic quantum number, (projection of angular momentum) | 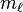 | energy shift (orientation of the subshell's shape) |  | for 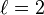 : : |
| spin projection quantum number |  | spin of the electron (-1/2 = counter-clockwise, 1/2 = clockwise) | 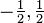 | for an electron, either: 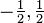 |
Note that molecular orbitals require totally different quantum numbers, because the Hamiltonian and its symmetries are quite different.
[edit] Quantum numbers with spin-orbit interaction
For more details on this topic, see Clebsch-Gordan coefficients.
When one takes the spin-orbit interaction into consideration, l, m and s no longer commute with the Hamiltonian, and their value therefore changes over time. Thus another set of quantum numbers should be used. This set includes- The total angular momentum quantum number (j = 1/2,3/2 ... n−1/2) gives the total angular momentum through the relation
 .
. - The projection of the total angular momentum along a specified axis (mj = -j,-j+1... j), which is analogous to m, and satisfies mj = ml + ms.
- Parity. This is the eigenvalue under reflection, and is positive (i.e. +1) for states which came from even l and negative (i.e. -1) for states which came from odd l. The former is also known as even parity and the latter as odd parity
- n = 2, l = 1, ml = 1, ms = +1/2
- n = 2, l = 1, ml = 1, ms = -1/2
- n = 2, l = 1, ml = 0, ms = +1/2
- n = 2, l = 1, ml = 0, ms = -1/2
- n = 2, l = 1, ml = -1, ms = +1/2
- n = 2, l = 1, ml = -1, ms = -1/2
- n = 2, l = 0, ml = 0, ms = +1/2
- n = 2, l = 0, ml = 0, ms = -1/2
- j = 3/2, mj = 3/2, odd parity (coming from state (1) above)
- j = 3/2, mj = 1/2, odd parity (coming from states (2) and (3) above)
- j = 3/2, mj = -1/2, odd parity (coming from states (4) and (5) above)
- j = 3/2, mj = -3/2, odd parity (coming from state (6))
- j = 1/2, mj = 1/2, odd parity (coming from states (2) and (3) above)
- j = 1/2, mj = -1/2, odd parity (coming from states (4) and (5) above)
- j = 1/2, mj = 1/2, even parity (coming from state (7) above)
- j = 1/2, mj = -1/2, even parity (coming from state (8) above)
[edit] Elementary particles
For a more complete description of the quantum states of elementary particles, see Standard model and Flavour (particle physics).
Elementary particles contain many quantum numbers which are usually said to be intrinsic to them. However, it should be understood that the elementary particles are quantum states of the standard model of particle physics, and hence the quantum numbers of these particles bear the same relation to the Hamiltonian of this model as the quantum numbers of the Bohr atom does to its Hamiltonian. In other words, each quantum number denotes a symmetry of the problem. It is more useful in field theory to distinguish between spacetime and internal symmetries.Typical quantum numbers related to spacetime symmetries are spin (related to rotational symmetry), the parity, C-parity and T-parity (related to the Poincare symmetry of spacetime). Typical internal symmetries are lepton number and baryon number or the electric charge. (For a full list of quantum numbers of this kind see the article on flavour.)
It is worth mentioning here a minor but often confusing point. Most conserved quantum numbers are additive. Thus, in an elementary particle reaction, the sum of the quantum numbers should be the same before and after the reaction. However, some, usually called a parity, are multiplicative; ie, their product is conserved. All multiplicative quantum numbers belong to a symmetry (like parity) in which applying the symmetry transformation twice is equivalent to doing nothing. These are all examples of an abstract group called Z2.
For more detailed explanation ClickHere!