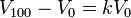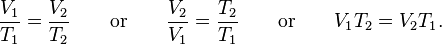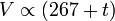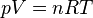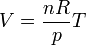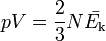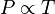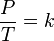The early gas laws were developed at the end of the eighteenth century, when scientists began to realize that relationships between the pressure, volume and temperature of a sample of gas could be obtained which would hold for all gases. Gases behave in a similar way over a wide variety of conditions because to a good approximation they all have molecules which are widely spaced, and nowadays the equation of state for an ideal gas is derived from kinetic theory. The earlier gas laws are now considered as special cases of the ideal gas equation, with one or more of the variables held constant.
Most gases behave like ideal gases at moderate pressures and temperatures. The limited technology of the 1600s could not produce high pressures or low temperatures. Hence, the law was not likely to have deviations at the time of publication. As improvements in technology permitted higher pressures and lower temperatures, deviations from the ideal gas behavior would become noticeable, and the relationship between pressure and volume can only be accurately described employing real gas theory.[7] The deviation is expressed as the compressibility factor.
Robert Boyle (and Edme Maria) derived the law solely on experimental grounds. The law can also be derived theoretically based on the presumed existence of atoms and molecules and assumptions about motion and perfectly elastic collisions (see kinetic theory of gases). These assumptions were met with enormous resistance in the positivist scientific community at the time however, as they were seen as purely theoretical constructs for which there was not the slightest observational evidence.
Daniel Bernoulli in 1738 derived Boyle's law using Newton's laws of motion with application on a molecular level. It remained ignored until around 1845, when John Waterston published a paper building the main precepts of kinetic theory; this was rejected by the Royal Society of England. Later works of James Prescott Joule, Rudolf Clausius and in particular Ludwig Boltzmann firmly established the kinetic theory of gases and brought attention to both the theories of Bernoulli and Waterston.[8]
The debate between proponents of Energetics and Atomism led Boltzmann to write a book in 1898, which endured criticism up to his suicide in 1906.[8] Albert Einstein in 1905 showed how kinetic theory applies to the Brownian motion of a fluid-suspended particle, which was confirmed in 1908 by Jean Perrin.[8]
Forcing the volume V of the fixed quantity of gas to increase, keeping the gas at the initially measured temperature, the pressure p must decrease proportionally. Conversely, reducing the volume of the gas increases the pressure.
Boyle's law is used to predict the result of introducing a change, in volume and pressure only, to the initial state of a fixed quantity of gas. The before and after volumes and pressures of the fixed amount of gas, where the before and after temperatures are the same (heating or cooling will be required to meet this condition), are related by the equation:
Whatever the priority of the discovery, Gay-Lussac was the first to demonstrate that the law applied generally to all gases, and also to the vapours of volatile liquids if the temperature was more than a few degrees above the boiling point. His statement of the law can be expressed mathematically as:
A modern statement of Charles's law is:
This law of volumes implies theoretically that as a temperature reaches absolute zero the gas will shrink down to zero volume. This is not physically correct, since in fact all gases turn into liquids at a low enough temperature, and Charles' law is not applicable at low temperatures for this reason.
The fact that the gas will occupy a non-zero volume - even as the temperature approaches absolute zero - arises fundamentally from the uncertainty principle of quantum theory. However, as the temperature is reduced, gases turn into liquids long before the limits of the uncertainty principle come into play due to the attractive forces between molecules which are neglected by Charles' Law.
The modern statement of the ideal gas law is:
An ideal gas is defined as a gas which obeys the ideal gas law, so Charles's law is only expected to be followed exactly by ideal gases. Nevertheless, it is a good approximation to the behaviour of real gases at relatively high temperatures and relatively low pressures.
For comparing the same substance under two different sets of conditions, the law can be written as:
Charles's Law was also known as the Law of Charles and Gay-Lussac, because Gay-Lussac published it in 1802 using much of Charles' unpublished data from 1787. However, in recent years the term has fallen out of favor since Gay-Lussac has the second but related law presented here and attributed to him. This related form of Gay-Lussac's Law, Charles's Law, and Boyle's law form the combined gas law. The three gas laws in combination with Avogadro's Law can be generalized by the ideal gas law.
As an example, equal volumes of molecular hydrogen and nitrogen would contain the same number of molecules, as long as they are at the same temperature and pressure and observe ideal or perfect gas behavior. In practice, for real gases, the law only holds approximately, but the agreement is close enough for the approximation to be useful.
The law can be stated mathematically as:
One mole of an ideal gas occupies 22.40 litres (dm³) at STP, and occupies 24.45 litres at SATP (Standard Ambient Temperature and Pressure = 298K and 1 atm). This volume is often referred to as the molar volume of an ideal gas. Real gases may deviate from this value.
Boyle's law
Boyle's law (sometimes referred to as the Boyle-Mariotte law) is one of several gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system.[1][2] The law was named after chemist and physicist Robert Boyle, who published the original law in 1662.[3] The law itself can be stated as follows:
For a fixed amount of an ideal gas kept at a fixed temperature, P [pressure] and V [volume] are inversely proportional (while one increases, the other decreases).[2]
History
This relationship between pressure and volume was first noted by two amateur scientists, Richard Towneley and Henry Power. Boyle confirmed their discovery through experiments and published the results. According to Robert Gunther and other authorities, it was Boyle's assistant Robert Hooke, who built the experimental apparatus. Boyle's law is based on experiments with air, which he considered to be a fluid of particles at rest, with in between small invisible springs. At that time air was still seen as one of the four elements, but Boyle didn't agree. Probably Boyle's interest was to understand air as an essential element of life [4]; he published e.g. the growth of plants without air [5]. The French physicist Edme Mariotte (1620-1684) discovered the same law independently of Boyle in 1676, but Boyle had already published it in 1662, so this law may, improperly, be referred to as Mariotte's or the Boyle-Mariotte law. Later (1687) in the Philosophiæ Naturalis Principia Mathematica Newton showed mathematically that if an elastic fluid consisting of particles at rest, between which are repulsive forces inversely proportional to their distance , the density would be proportional to the pressure [6], but this mathematical treatise is not the physical explanation for the observed relationship. Instead of a static theory a kinetic theory is needed, which was provided two centuries later by Maxwell and Boltzmann.Definition
Relation to kinetic theory and ideal gases
Boyle’s law states that at constant temperature for a fixed mass, the absolute pressure and the volume of a gas are inversely proportional. The law can also be stated in a slightly different manner, that the product of absolute pressure and volume is always constant.Most gases behave like ideal gases at moderate pressures and temperatures. The limited technology of the 1600s could not produce high pressures or low temperatures. Hence, the law was not likely to have deviations at the time of publication. As improvements in technology permitted higher pressures and lower temperatures, deviations from the ideal gas behavior would become noticeable, and the relationship between pressure and volume can only be accurately described employing real gas theory.[7] The deviation is expressed as the compressibility factor.
Robert Boyle (and Edme Maria) derived the law solely on experimental grounds. The law can also be derived theoretically based on the presumed existence of atoms and molecules and assumptions about motion and perfectly elastic collisions (see kinetic theory of gases). These assumptions were met with enormous resistance in the positivist scientific community at the time however, as they were seen as purely theoretical constructs for which there was not the slightest observational evidence.
Daniel Bernoulli in 1738 derived Boyle's law using Newton's laws of motion with application on a molecular level. It remained ignored until around 1845, when John Waterston published a paper building the main precepts of kinetic theory; this was rejected by the Royal Society of England. Later works of James Prescott Joule, Rudolf Clausius and in particular Ludwig Boltzmann firmly established the kinetic theory of gases and brought attention to both the theories of Bernoulli and Waterston.[8]
The debate between proponents of Energetics and Atomism led Boltzmann to write a book in 1898, which endured criticism up to his suicide in 1906.[8] Albert Einstein in 1905 showed how kinetic theory applies to the Brownian motion of a fluid-suspended particle, which was confirmed in 1908 by Jean Perrin.[8]
Equation
The mathematical equation for Boyle's law is:- p denotes the pressure of the system.
- V is the volume of the gas.
- k is a constant value representative of the pressure and volume of the system.
Forcing the volume V of the fixed quantity of gas to increase, keeping the gas at the initially measured temperature, the pressure p must decrease proportionally. Conversely, reducing the volume of the gas increases the pressure.
Boyle's law is used to predict the result of introducing a change, in volume and pressure only, to the initial state of a fixed quantity of gas. The before and after volumes and pressures of the fixed amount of gas, where the before and after temperatures are the same (heating or cooling will be required to meet this condition), are related by the equation:
Charles's law
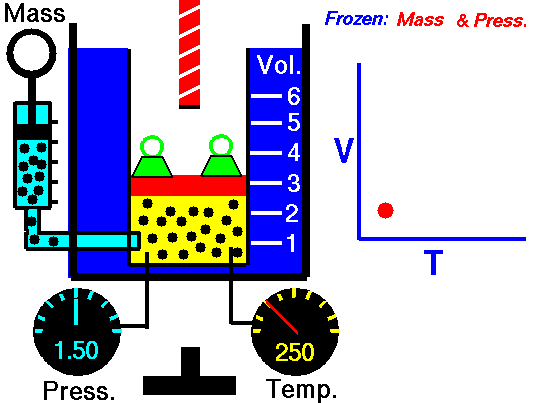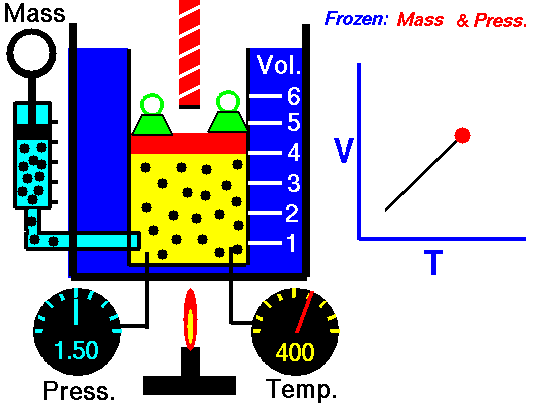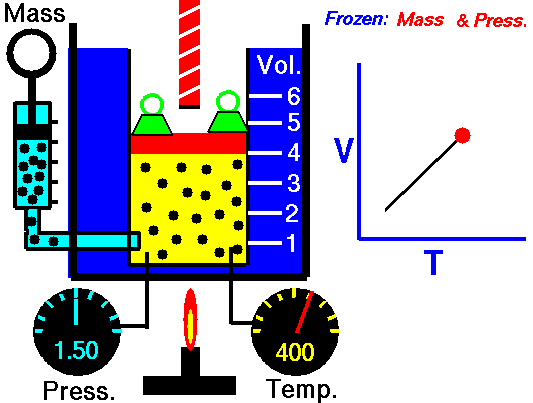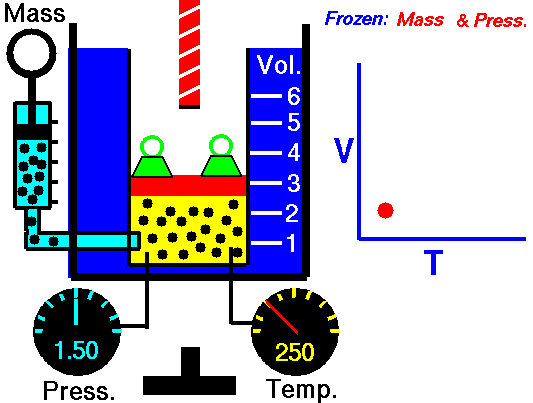
Charles's law (also known as the law of volumes) is an experimental gas law which describes how gasses tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802,[1] although he credits the discovery to unpublished work from the 1780s by Jacques Charles. The law was independently discovered by British natural philosopher John Dalton by 1801, although Dalton's description was less thorough than Gay-Lussac's. The basic principles had already been described a century earlier by Guillaume Amontons.Whatever the priority of the discovery, Gay-Lussac was the first to demonstrate that the law applied generally to all gases, and also to the vapours of volatile liquids if the temperature was more than a few degrees above the boiling point. His statement of the law can be expressed mathematically as:
A modern statement of Charles's law is:
At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. the gas expands as the temperature increases).[2]which can be written as:
Limitations
In modern physics, Charles's Law is seen as a special case of the ideal gas equation, in which the pressure and number of molecules are held constant. The ideal gas equation is usually derived from the kinetic theory of gases, which presumes that molecules occupy negligible volume, do not attract each other and undergo elastic collisions (no loss of kinetic energy); an imaginary gas with exactly these properties is termed an ideal gas. The behavior of a real gas is close to that of an ideal gas under most circumstances, which makes the ideal gas law useful.This law of volumes implies theoretically that as a temperature reaches absolute zero the gas will shrink down to zero volume. This is not physically correct, since in fact all gases turn into liquids at a low enough temperature, and Charles' law is not applicable at low temperatures for this reason.
The fact that the gas will occupy a non-zero volume - even as the temperature approaches absolute zero - arises fundamentally from the uncertainty principle of quantum theory. However, as the temperature is reduced, gases turn into liquids long before the limits of the uncertainty principle come into play due to the attractive forces between molecules which are neglected by Charles' Law.
Relation to the ideal gas law
French physicist Émile Clapeyron combined Charles's law with Boyle's law in 1834 to produce a single statement which would become known as the ideal gas law.[3] Claypeyron's original statement was:The modern statement of the ideal gas law is:
An ideal gas is defined as a gas which obeys the ideal gas law, so Charles's law is only expected to be followed exactly by ideal gases. Nevertheless, it is a good approximation to the behaviour of real gases at relatively high temperatures and relatively low pressures.
Relation to absolute zero
Charles's law appears to imply that the volume of a gas will descend to zero at a certain temperature (−266.66 ºC according to Gay-Lussac's figures). Gay-Lussac was clear in his description that the law was not applicable at low temperatures:but I may mention that this last conclusion cannot be true except so long as the compressed vapors remain entirely in the elastic state; and this requires that their temperature shall be sufficiently elevated to enable them to resist the pressure which tends to make them assume the liquid state.[1]Gay-Lussac had no experience of liquid air (first prepared in 1877), although he appears to believe (as did Dalton) that the "permanent gases" such as air and hydrogen could be liquified. Gay-Lussac had also worked with the vapours of volatile liquids in demonstrating Charles's law, and was aware that the law does not apply just above the boiling point of the liquid:
I may however remark that when the temperature of the ether is only a little above its boiling point, its condensation is a little more rapid than that of atmospheric air. This fact is related to a phenomenon which is exhibited by a great many bodies when passing from the liquid to the solid state, but which is no longer sensible at temperatures a few degrees above that at which the transition occurs.[1]The first mention of a temperature at which the volume of a gas might descend to zero was by William Thomson (later known as Lord Kelvin) in 1848:[4]
This is what we might anticipate, when we reflect that infinite cold must correspond to a finite number of degrees of the air-thermometer below zero; since if we push the strict principle of graduation, stated above, sufficiently far, we should arrive at a point corresponding to the volume of air being reduced to nothing, which would be marked as −273° of the scale (−100/.366, if .366 be the coefficient of expansion); and therefore −273° of the air-thermometer is a point which cannot be reached at any finite temperature, however low.However, the "absolute zero" on the Kelvin temperature scale was originally defined in terms of the second law of thermodynamics, which Thomson himself described in 1852.[5] Thomson did not assume that this was equal to the "zero-volume point" of Charles's law, merely that Charles's law provided the minimum temperature which could be attained. The two can be shown to be equivalent by Ludwig Boltzmann's statistical view of entropy (1870).
Relation to kinetic theory
The kinetic theory of gases relates the macroscopic properties of gases, such as pressure and volume, to the microscopic properties of the molecules which make up the gas, particularly the mass and speed of the molecules. In order to derive Charles's law from kinetic theory, it is necessary to have a microscopic definition of temperature: this can be conveniently taken as the temperature being proportional to the average kinetic energy of the gas molecules, Ek:Gay-Lussac's law
The expression Gay-Lussac's law is used for each of the two relationships named after the French chemist Joseph Louis Gay-Lussac and which concern the properties of gases, though it is more usually applied to his law of combining volumes, the first listed here. One law relates to volumes before and after a chemical reaction while the other concerns the pressure and temperature relationship for a sample of gas.Law of combining volumes
The law of combining volumes states that, when gases react together to form other gases, and all volumes are measured at the same temperature and pressure:The ratio between the volumes of the reactant gases and the products can be expressed in simple whole numbers.This reflects the fact that (by Avogadro's law) equal volumes of gas contain equal numbers of molecules (at the same temperature and pressure), and also that in chemical reactions, the molecules combine in a ratio of whole numbers - this is known as the stoichiometry of the chemical reaction and is expressed via the chemical equation for the reaction.
Pressure-temperature law
Gay-Lussac's other law, discovered in 1802, states that:The pressure of a fixed mass and fixed volume of a gas is directly proportional to the gas's temperature.Simply put, if a gas's temperature increases then so does its pressure, if the mass and volume of the gas are held constant. The law has a particularly simple mathematical form if the temperature is measured on an absolute scale, such as in Kelvin. The law can then be expressed mathematically as:
- P is the pressure of the gas.
- T is the temperature of the gas (measured in Kelvin).
- k is a constant.
For comparing the same substance under two different sets of conditions, the law can be written as:
Charles's Law was also known as the Law of Charles and Gay-Lussac, because Gay-Lussac published it in 1802 using much of Charles' unpublished data from 1787. However, in recent years the term has fallen out of favor since Gay-Lussac has the second but related law presented here and attributed to him. This related form of Gay-Lussac's Law, Charles's Law, and Boyle's law form the combined gas law. The three gas laws in combination with Avogadro's Law can be generalized by the ideal gas law.
Avogadro's law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) is a gas law named after Amedeo Avogadro who, in 1811,[1] hypothesized that "Equal volumes of ideal or perfect gases, at the same temperature and pressure, contain the same number of particles, or molecules." Thus, the number of molecules in a specific volume of gas is independent of the size or mass of the gas molecules.As an example, equal volumes of molecular hydrogen and nitrogen would contain the same number of molecules, as long as they are at the same temperature and pressure and observe ideal or perfect gas behavior. In practice, for real gases, the law only holds approximately, but the agreement is close enough for the approximation to be useful.
The law can be stated mathematically as:
 .
.
- V is the volume of the gas.
- n is the amount of substance of the gas.
- k is a proportionality constant.
- p is the pressure of the gas
- T is the temperature of the gas
One mole of an ideal gas occupies 22.40 litres (dm³) at STP, and occupies 24.45 litres at SATP (Standard Ambient Temperature and Pressure = 298K and 1 atm). This volume is often referred to as the molar volume of an ideal gas. Real gases may deviate from this value.