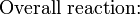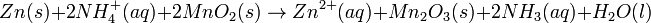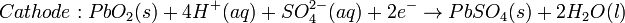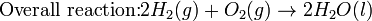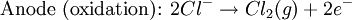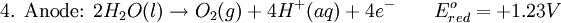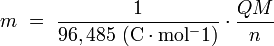Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductor) and an ionic conductor (the electrolyte), and which involve electron transfer between the electrode and the electrolyte or species in solution.
If a chemical reaction is driven by an external applied voltage, as in electrolysis, or if a voltage is created by a chemical reaction as in a battery, it is an electrochemical reaction. Chemical reactions where electrons are transferred between molecules are called oxidation/reduction (redox) reactions. In general, electrochemistry deals with situations where oxidation and reduction reactions are separated in space or time, connected by an external electric circuit to understand each process.
For example, when atomic sodium reacts with atomic chlorine, sodium donates one electron and attains an oxidation state of +1. Chlorine accepts the electron and its oxidation state is reduced to −1. The sign of the oxidation state (positive/negative) actually corresponds to the value of each ion's electronic charge. The attraction of the differently charged sodium and chlorine ions is the reason they then form an ionic bond.
The loss of electrons from an atom or molecule is called oxidation, and the gain of electrons is reduction. This can be easily remembered through the use of mnemonic devices. Two of the most popular are "OIL RIG" (Oxidation Is Loss, Reduction Is Gain) and "LEO" the lion says "GER" (Lose Electrons: Oxidization, Gain Electrons: Reduction). For cases where electrons are shared (covalent bonds) between atoms with large differences in electronegativity, the electron is assigned to the atom with the largest electronegativity in determining the oxidation state.
The atom or molecule which loses electrons is known as the reducing agent, or reductant, and the substance which accepts the electrons is called the oxidizing agent, or oxidant. The oxidizing agent is always being reduced in a reaction; the reducing agent is always being oxidized. Oxygen is a common oxidizing agent, but not the only one. Despite the name, an oxidation reaction does not necessarily need to involve oxygen. In fact, a fire can be fed by an oxidant other than oxygen; fluorine fires are often unquenchable, as fluorine is an even stronger oxidant (it has a higher electronegativity) than oxygen.
For reactions involving oxygen, the gain of oxygen implies the oxidation of the atom or molecule to which the oxygen is added (and the oxygen is reduced). For example, in the oxidation of octane by oxygen to form carbon dioxide and water, both the carbon in the octane and the oxygen begin with an oxidation state of 0. In forming CO2 the carbon loses four electrons to become C4+ and the oxygens each gain two electrons to be O2-. In organic compounds, such as butane or ethanol, the loss of hydrogen implies oxidation of the molecule from which it is lost (and the hydrogen is reduced). This follows because the hydrogen donates its electron in covalent bonds with non-metals but it takes the electron along when it is lost. Conversely, loss of oxygen or gain of hydrogen implies reduction.
Electrochemical cells have two conductive electrodes (the anode and the cathode). The anode is defined as the electrode where oxidation occurs and the cathode is the electrode where the reduction takes place. Electrodes can be made from any sufficiently conductive materials, such as metals, semiconductors, graphite, and even conductive polymers. In between these electrodes is the electrolyte, which contains ions that can freely move.
The Galvanic cell uses two different metal electrodes, each in an electrolyte where the positively charged ions are the oxidized form of the electrode metal. One electrode will undergo oxidation (the anode) and the other will undergo reduction (the cathode). The metal of the anode will oxidize, going from an oxidation state of 0 (in the solid form) to a positive oxidation state and become an ion. At the cathode, the metal ion in solution will accept one or more electrons from the cathode and the ion's oxidation state is reduced to 0. This forms a solid metal that electrodeposits on the cathode. The two electrodes must be electrically connected to each other, allowing for a flow of electrons that leave the metal of the anode and flow through this connection to the ions at the surface of the cathode. This flow of electrons is an electrical current that can be used to do work, such as turn a motor or power a light.
A Galvanic cell whose electrodes are zinc and copper submerged in zinc sulfate and copper sulfate, respectively, is known as a Daniell cell.
Half reactions for a Daniell cell are these:
 In this example, the anode is zinc metal which oxidizes (loses electrons) to form zinc ions in solution, and copper ions accept electrons from the copper metal electrode and the ions deposit at the copper cathode as an electrodeposit. This cell forms a simple battery as it will spontaneously generate a flow of electrical current from the anode to the cathode through the external connection. This reaction can be driven in reverse by applying a voltage, resulting in the deposition of zinc metal at the anode and formation of copper ions at the cathode.
In this example, the anode is zinc metal which oxidizes (loses electrons) to form zinc ions in solution, and copper ions accept electrons from the copper metal electrode and the ions deposit at the copper cathode as an electrodeposit. This cell forms a simple battery as it will spontaneously generate a flow of electrical current from the anode to the cathode through the external connection. This reaction can be driven in reverse by applying a voltage, resulting in the deposition of zinc metal at the anode and formation of copper ions at the cathode.
To provide a complete electric circuit, there must also be an ionic conduction path between the anode and cathode electrolytes in addition to the electron conduction path. The simplest ionic conduction path is to provide a liquid junction. To avoid mixing between the two electrolytes, the liquid junction can be provided through a porous plug that allows ion flow while reducing electrolyte mixing. To further minimize mixing of the electrolytes, a salt bridge can be used which consists of an electrolyte saturated gel in an inverted U-tube. As the negatively charged electrons flow in one direction around this circuit, the positively charged metal ions flow in the opposite direction in the electrolyte.
A voltmeter is capable of measuring the change of electrical potential between the anode and the cathode.
Electrochemical cell voltage is also referred to as electromotive force or emf.
A cell diagram can be used to trace the path of the electrons in the electrochemical cell. For example, here is a cell diagram of a Daniell cell:
The SHE electrode can be connected to any other electrode by a salt bridge to form a cell. If the second electrode is also at standard conditions, then the measured cell potential is called the standard electrode potential for the electrode. The standard electrode potential for the SHE is zero, by definition. The polarity of the standard electrode potential provides information about the relative reduction potential of the electrode compared to the SHE. If the electrode has a positive potential with respect to the SHE, then that means it is a strongly reducing electrode which forces the SHE to be the anode (an example is Cu in aqueous CuSO4 with a standard electrode potential of 0.337 V). Conversely, if the measured potential is negative, the electrode is more oxidizing than the SHE (such as Zn in ZnSO4 where the standard electrode potential is -0.763 V).
Standard electrode potentials are usually tabulated as reduction potentials. However, the reactions are reversible and the role of a particular electrode in a cell depends on the relative oxidation/reduction potential of both electrodes. The oxidation potential for a particular electrode is just the negative of the reduction potential. A standard cell potential can be determined by looking up the standard electrode potentials for both electrodes (sometimes called half cell potentials). The one that is smaller will be the anode and will undergo oxidation. The cell potential is then calculated as the sum of the reduction potential for the cathode and the oxidation potential for the anode.
 value because the standard electrode potential is an intensive property.
value because the standard electrode potential is an intensive property.
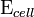 is the cell potential measured in volts (V) and
is the cell potential measured in volts (V) and  is the cell current integrated over time and measured in coulombs (C).
is the cell current integrated over time and measured in coulombs (C).  can also be determined by multiplying the total number of electrons transferred (measured in moles) times Faraday's constant(F).
can also be determined by multiplying the total number of electrons transferred (measured in moles) times Faraday's constant(F).
The emf of the cell at zero current is the maximum possible emf. It is used to calculate the maximum possible electrical energy that could be obtained from a chemical reaction. This energy is referred to as electrical work and is expressed by the following equation:
Since the free energy is the maximum amount of work that can be extracted from a system, one can write:
A spontaneous electrochemical reaction (change in Gibbs free energy less than zero) can be used to generate an electric current, in electrochemical cells. This is the basis of all batteries and fuel cells. For example, gaseous oxygen (O2) and hydrogen (H2) can be combined in a fuel cell to form water and energy, typically a combination of heat and electrical energy.
Conversely, non-spontaneous electrochemical reactions can be driven forward by the application of a current at sufficient voltage. The electrolysis of water into gaseous oxygen and hydrogen is a typical example.
The relation between the equilibrium constant, K, and the Gibbs free energy for an electrochemical cell is expressed as follows:
In the late 19th century Josiah Willard Gibbs had formulated a theory to predict whether a chemical reaction is spontaneous based on the free energy
ΔG = change in Gibbs free energy, T = absolute temperature, R = gas constant, ln = natural logarithm, Q = reaction quotient.
Gibbs' key contribution was to formalize the understanding of the effect of reactant concentration on spontaneity.
Based on Gibbs' work, Nernst extended the theory to include the contribution from electric potential on charged species. As shown in the previous section, the change in Gibbs free energy for an electrochemical cell can be related to the cell potential. Thus, Gibbs' theory becomes
n = number of electrons/mole product, F = Faraday constant (coulombs/mole), and ΔE = cell potential.
Finally, Nernst divided through by the amount of charge transferred to arrive at a new equation which now bears his name:
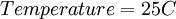 ) and R =
) and R =  the equation above can be expressed on Base—10 logarithm as shown below:
the equation above can be expressed on Base—10 logarithm as shown below:
For example an electrochemical cell, where two copper electrodes are submerged in two copper(II) sulfate solutions, whose concentrations are 0.05 M and 2.0 M, connected through a salt bridge. This type of cell will generate a potential that can be predicted by the Nernst equation. Both electrodes undergo the same chemistry (although the reaction proceeds in reverse at the cathode)
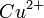 ions increases. Reduction will take place in the cell's compartment where concentration is higher and oxidation will occur on the more dilute side.
ions increases. Reduction will take place in the cell's compartment where concentration is higher and oxidation will occur on the more dilute side.
The following cell diagram describes the cell mentioned above:
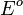 's value of this kind of cell is zero, as electrodes and ions are the same in both half-cells. After replacing values from the case mentioned, it is possible to calculate cell's potential:
's value of this kind of cell is zero, as electrodes and ions are the same in both half-cells. After replacing values from the case mentioned, it is possible to calculate cell's potential:
The Nernst equation plays an important role in understanding electrical effects in cells and organelles. Such effects include nerve synapses and cardiac beat as well as the resting potential of a somatic cell.
Leclanché's simplified half reactions are shown below:
Mercury battery half reactions are shown below:
Lead-acid battery half cell reactions are shown below:

At standard conditions, each cell may produce a potential of 2 V, hence overall voltage produced is 12 V. Differing from mercury and zinc-carbon batteries, lead-acid batteries are rechargeable. If an external voltage is supplied to the battery it will produce an electrolysis of the products in the overall reaction (discharge), thus recovering initial components which made the battery work.
These types of batteries are typically used for large-scale energy storage (kWh - multi MWh). Of the several different types that have been developed, some are of current commercial interest, including the iron/chromium flow battery, vanadium redox battery and zinc-bromine flow battery.
To enhance electrical production, scientists have developed fuel cells where combustion is replaced by electrochemical methods, similar to a battery but requiring continuous replenishment of the reactants consumed.
The most popular is the oxygen-hydrogen fuel cell, where two inert electrodes (porous electrodes of nickel and nickel oxide [disambiguation needed]) are placed in an electrolytic solution such as hot caustic potash, in both compartments (anode and cathode) gaseous hydrogen and oxygen are bubbled into solution.
Oxygen-hydrogen fuel cell reactions are shown bellow:
 . The electric circuit works as passage of electrons and ions occurs, thus if an electrolyte is present it will facilitate oxidation, this explains why rusting is quicker on salt water.
. The electric circuit works as passage of electrons and ions occurs, thus if an electrolyte is present it will facilitate oxidation, this explains why rusting is quicker on salt water.
Gold and platinum are extremely difficult to oxidize under normal circumstances, and require exposure to a powerful chemical oxidizing agent such as aqua regia.
Some common metals oxidize extremely rapidly in air. Titanium and aluminium oxidize instantaneouly in contact with the oxygen in the air. These metals form an extremely thin layer of oxidized metal on the surface. This thin layer of oxide protects the underlying layers of the metal from the air preventing the entire metal from oxidizing. These metals are used in applications where corrosion resistance is important. Iron, in contrast, has an oxide that forms in air and water, called rust, that does not stop the further oxidation of the iron. Thus iron left exposed to air and water will continue to rust until all of the iron is oxidized.
While it is almost impossible to prevent anode/cathode formation, if a non-conducting material covers the metal, contact with the electrolyte is not possible and corrosion will not occur.
Zinc bars are attached at various locations on steel ship hulls to render the ship hull cathodic. The zinc bars are replaced periodically. Other metals, such as magnesium, would work very well but zinc is the least expensive useful metal.
To protect pipelines, an ingot of buried or exposed magnesium (or zinc) is buried beside the pipeline and is connected electrically to the pipe above ground. The pipeline is forced to be a cathode and is protected from being oxidized and rusting. The magnesium anode is sacrificed. At intervals new ingots are buried to replace those lost.
Reactions that take place at Down's cell are the following:
The emf [disambiguation needed] for this process is approximately -4 V indicating a (very) non-spontaneous process. In order for this reaction to occur the power supply should provide at least a potential of 4 V. However, larger voltages must be used for this reaction to occur at a high rate.
Bubbles from the gases will be seen near both electrodes. The following half reactions describe the process mentioned above:
The following half reactions describes the process mentioned:
When comparing the reduction potentials in reactions 2 & 4, the reduction of chloride ion is favored. Thus, if the Cl- ion is favored for reduction, then the water reaction is favored for oxidation producing gaseous oxygen, however experiments show gaseous chlorine is produced and not oxygen.
Although the initial analysis is correct, there is another effect that can happen, known as the overvoltage effect. Additional voltage is sometimes required, beyond the voltage predicted by the . This may be due to kinetic rather than thermodynamic considerations. In fact, it has been proven that the activation energy for the chloride ion is very low, hence favorable in kinetic terms. In other words, although the voltage applied is thermodynamically sufficient to drive electrolysis, the rate is so slow that to make the process proceed in a reasonable time frame, the voltage of the external source has to be increased (hence, overvoltage).
. This may be due to kinetic rather than thermodynamic considerations. In fact, it has been proven that the activation energy for the chloride ion is very low, hence favorable in kinetic terms. In other words, although the voltage applied is thermodynamically sufficient to drive electrolysis, the rate is so slow that to make the process proceed in a reasonable time frame, the voltage of the external source has to be increased (hence, overvoltage).
Finally, reaction 3 is favorable because it describes the proliferation of OH- ions thus letting a probable reduction of H+ ions less favorable an option.
The overall reaction for the process according to the analysis would be the following:
In other words, the amount of a substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity passed through the cell.
Below a simplified equation of Faraday's first law:
An important aspect of the second law of electrolysis is electroplating which together with the first law of electrolysis, has a significant number of applications in the industry, as when used to protect metals to avoid corrosion.
The nervous impulses in neurons are based on electric current generated by the movement of sodium and potassium ions into and out of cells, and certain animals like eels can generate a powerful voltage from certain cells that can disable much larger animals.
Main Articles:
Electroplating
Faraday's law of electrolysis
Redox reaction
Corrosion
Electrochemical cell
Standard electrode potential
If a chemical reaction is driven by an external applied voltage, as in electrolysis, or if a voltage is created by a chemical reaction as in a battery, it is an electrochemical reaction. Chemical reactions where electrons are transferred between molecules are called oxidation/reduction (redox) reactions. In general, electrochemistry deals with situations where oxidation and reduction reactions are separated in space or time, connected by an external electric circuit to understand each process.
Principles
Redox reactions
Redox stands for reduction-oxidation, and are electrochemical processes involving electron transfer to or from a molecule or ion changing its oxidation state. This reaction can occur through the application of an external voltage or through the release of chemical energy.Oxidation and reduction
Oxidation and reduction describe the change of oxidation state that takes place in the atoms, ions or molecules involved in an electrochemical reaction. Formally, oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. An atom or ion that gives up an electron to another atom or ion has its oxidation state increase, and the recipient of the negatively charged electron has its oxidation state decrease. Oxidation and reduction always occur in a paired fashion such that one species is oxidized when another is reduced. This paired electron transfer is called a redox reaction.For example, when atomic sodium reacts with atomic chlorine, sodium donates one electron and attains an oxidation state of +1. Chlorine accepts the electron and its oxidation state is reduced to −1. The sign of the oxidation state (positive/negative) actually corresponds to the value of each ion's electronic charge. The attraction of the differently charged sodium and chlorine ions is the reason they then form an ionic bond.
The loss of electrons from an atom or molecule is called oxidation, and the gain of electrons is reduction. This can be easily remembered through the use of mnemonic devices. Two of the most popular are "OIL RIG" (Oxidation Is Loss, Reduction Is Gain) and "LEO" the lion says "GER" (Lose Electrons: Oxidization, Gain Electrons: Reduction). For cases where electrons are shared (covalent bonds) between atoms with large differences in electronegativity, the electron is assigned to the atom with the largest electronegativity in determining the oxidation state.
The atom or molecule which loses electrons is known as the reducing agent, or reductant, and the substance which accepts the electrons is called the oxidizing agent, or oxidant. The oxidizing agent is always being reduced in a reaction; the reducing agent is always being oxidized. Oxygen is a common oxidizing agent, but not the only one. Despite the name, an oxidation reaction does not necessarily need to involve oxygen. In fact, a fire can be fed by an oxidant other than oxygen; fluorine fires are often unquenchable, as fluorine is an even stronger oxidant (it has a higher electronegativity) than oxygen.
For reactions involving oxygen, the gain of oxygen implies the oxidation of the atom or molecule to which the oxygen is added (and the oxygen is reduced). For example, in the oxidation of octane by oxygen to form carbon dioxide and water, both the carbon in the octane and the oxygen begin with an oxidation state of 0. In forming CO2 the carbon loses four electrons to become C4+ and the oxygens each gain two electrons to be O2-. In organic compounds, such as butane or ethanol, the loss of hydrogen implies oxidation of the molecule from which it is lost (and the hydrogen is reduced). This follows because the hydrogen donates its electron in covalent bonds with non-metals but it takes the electron along when it is lost. Conversely, loss of oxygen or gain of hydrogen implies reduction.
Balancing redox reactions
Electrochemical reactions in water are better understood by balancing redox reactions using the Ion-Electron Method where H+ , OH- ion, H2O and electrons (to compensate the oxidation changes) are added to cell's half-reactions for oxidation and reduction.Acidic medium
In acid medium H+ ions and water are added to half-reactions to balance the overall reaction. For example, when manganese reacts with sodium bismuthate.Basic medium
In basic medium OH- ions and water are added to half reactions to balance the overall reaction. For example on reaction between Potassium permanganate and Sodium sulfite.Neutral medium
The same procedure as used on acid medium is applied, for example on balancing using electron ion method to complete combustion of propane.Electrochemical cells
An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the Galvanic cell or Voltaic cell [disambiguation needed], named after Luigi Galvani and Alessandro Volta, both scientists who conducted several experiments on chemical reactions and electric current during the late 18th century.Electrochemical cells have two conductive electrodes (the anode and the cathode). The anode is defined as the electrode where oxidation occurs and the cathode is the electrode where the reduction takes place. Electrodes can be made from any sufficiently conductive materials, such as metals, semiconductors, graphite, and even conductive polymers. In between these electrodes is the electrolyte, which contains ions that can freely move.
The Galvanic cell uses two different metal electrodes, each in an electrolyte where the positively charged ions are the oxidized form of the electrode metal. One electrode will undergo oxidation (the anode) and the other will undergo reduction (the cathode). The metal of the anode will oxidize, going from an oxidation state of 0 (in the solid form) to a positive oxidation state and become an ion. At the cathode, the metal ion in solution will accept one or more electrons from the cathode and the ion's oxidation state is reduced to 0. This forms a solid metal that electrodeposits on the cathode. The two electrodes must be electrically connected to each other, allowing for a flow of electrons that leave the metal of the anode and flow through this connection to the ions at the surface of the cathode. This flow of electrons is an electrical current that can be used to do work, such as turn a motor or power a light.
A Galvanic cell whose electrodes are zinc and copper submerged in zinc sulfate and copper sulfate, respectively, is known as a Daniell cell.
Half reactions for a Daniell cell are these:

A modern cell stand for electrochemical research. The electrodes attach to high-quality metallic wires, and the stand is attached to a potentiostat/galvanostat (not pictured). A shot glass-shaped container is aerated with a noble gas and sealed with the Teflon block.
To provide a complete electric circuit, there must also be an ionic conduction path between the anode and cathode electrolytes in addition to the electron conduction path. The simplest ionic conduction path is to provide a liquid junction. To avoid mixing between the two electrolytes, the liquid junction can be provided through a porous plug that allows ion flow while reducing electrolyte mixing. To further minimize mixing of the electrolytes, a salt bridge can be used which consists of an electrolyte saturated gel in an inverted U-tube. As the negatively charged electrons flow in one direction around this circuit, the positively charged metal ions flow in the opposite direction in the electrolyte.
A voltmeter is capable of measuring the change of electrical potential between the anode and the cathode.
Electrochemical cell voltage is also referred to as electromotive force or emf.
A cell diagram can be used to trace the path of the electrons in the electrochemical cell. For example, here is a cell diagram of a Daniell cell:
[edit] Standard electrode potential
To allow prediction of the cell potential, tabulations of standard electrode potential are available. Such tabulations are referenced to the standard hydrogen electrode (SHE). The standard hydrogen electrode undergoes the reactionThe SHE electrode can be connected to any other electrode by a salt bridge to form a cell. If the second electrode is also at standard conditions, then the measured cell potential is called the standard electrode potential for the electrode. The standard electrode potential for the SHE is zero, by definition. The polarity of the standard electrode potential provides information about the relative reduction potential of the electrode compared to the SHE. If the electrode has a positive potential with respect to the SHE, then that means it is a strongly reducing electrode which forces the SHE to be the anode (an example is Cu in aqueous CuSO4 with a standard electrode potential of 0.337 V). Conversely, if the measured potential is negative, the electrode is more oxidizing than the SHE (such as Zn in ZnSO4 where the standard electrode potential is -0.763 V).
Standard electrode potentials are usually tabulated as reduction potentials. However, the reactions are reversible and the role of a particular electrode in a cell depends on the relative oxidation/reduction potential of both electrodes. The oxidation potential for a particular electrode is just the negative of the reduction potential. A standard cell potential can be determined by looking up the standard electrode potentials for both electrodes (sometimes called half cell potentials). The one that is smaller will be the anode and will undergo oxidation. The cell potential is then calculated as the sum of the reduction potential for the cathode and the oxidation potential for the anode.
 value because the standard electrode potential is an intensive property.
value because the standard electrode potential is an intensive property.Spontaneity of Redox reaction
During operation of electrochemical cells, chemical energy is transformed into electrical energy and is expressed mathematically as the product of the cell's emf and the electrical charge transferred through the external circuit.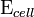 is the cell potential measured in volts (V) and
is the cell potential measured in volts (V) and  is the cell current integrated over time and measured in coulombs (C).
is the cell current integrated over time and measured in coulombs (C).  can also be determined by multiplying the total number of electrons transferred (measured in moles) times Faraday's constant(F).
can also be determined by multiplying the total number of electrons transferred (measured in moles) times Faraday's constant(F).The emf of the cell at zero current is the maximum possible emf. It is used to calculate the maximum possible electrical energy that could be obtained from a chemical reaction. This energy is referred to as electrical work and is expressed by the following equation:
Since the free energy is the maximum amount of work that can be extracted from a system, one can write:
A spontaneous electrochemical reaction (change in Gibbs free energy less than zero) can be used to generate an electric current, in electrochemical cells. This is the basis of all batteries and fuel cells. For example, gaseous oxygen (O2) and hydrogen (H2) can be combined in a fuel cell to form water and energy, typically a combination of heat and electrical energy.
Conversely, non-spontaneous electrochemical reactions can be driven forward by the application of a current at sufficient voltage. The electrolysis of water into gaseous oxygen and hydrogen is a typical example.
The relation between the equilibrium constant, K, and the Gibbs free energy for an electrochemical cell is expressed as follows:
Cell emf dependency on changes in concentration
Nernst Equation
The standard potential of an electrochemical cell requires standard conditions for all of the reactants. When reactant concentrations differ from standard conditions, the cell potential will deviate from the standard potential. In the 20th century German chemist Walther Nernst proposed a mathematical model to determine the effect of reactant concentration on electrochemical cell potential.In the late 19th century Josiah Willard Gibbs had formulated a theory to predict whether a chemical reaction is spontaneous based on the free energy
 ,
,
ΔG = change in Gibbs free energy, T = absolute temperature, R = gas constant, ln = natural logarithm, Q = reaction quotient.
Gibbs' key contribution was to formalize the understanding of the effect of reactant concentration on spontaneity.
Based on Gibbs' work, Nernst extended the theory to include the contribution from electric potential on charged species. As shown in the previous section, the change in Gibbs free energy for an electrochemical cell can be related to the cell potential. Thus, Gibbs' theory becomes
n = number of electrons/mole product, F = Faraday constant (coulombs/mole), and ΔE = cell potential.
Finally, Nernst divided through by the amount of charge transferred to arrive at a new equation which now bears his name:
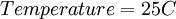 ) and R =
) and R =  the equation above can be expressed on Base—10 logarithm as shown below:
the equation above can be expressed on Base—10 logarithm as shown below:Concentration cells
A concentration cell is an electrochemical cell where the two electrodes are the same material, the electrolytes on the two half-cells involve the same ions, but the electrolyte concentration differs between the two half-cells.For example an electrochemical cell, where two copper electrodes are submerged in two copper(II) sulfate solutions, whose concentrations are 0.05 M and 2.0 M, connected through a salt bridge. This type of cell will generate a potential that can be predicted by the Nernst equation. Both electrodes undergo the same chemistry (although the reaction proceeds in reverse at the cathode)
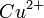 ions increases. Reduction will take place in the cell's compartment where concentration is higher and oxidation will occur on the more dilute side.
ions increases. Reduction will take place in the cell's compartment where concentration is higher and oxidation will occur on the more dilute side.The following cell diagram describes the cell mentioned above:
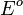 's value of this kind of cell is zero, as electrodes and ions are the same in both half-cells. After replacing values from the case mentioned, it is possible to calculate cell's potential:
's value of this kind of cell is zero, as electrodes and ions are the same in both half-cells. After replacing values from the case mentioned, it is possible to calculate cell's potential:The Nernst equation plays an important role in understanding electrical effects in cells and organelles. Such effects include nerve synapses and cardiac beat as well as the resting potential of a somatic cell.
Battery
A battery is an electrochemical cell (sometimes several in series) used for chemical energy storage. Batteries are optimized to produce a constant electric current for as long as possible. Although the cells discussed previously are useful for theoretical purposes and some laboratory experiments, the large internal resistance of the salt bridge make them inappropriate battery technologies. Various alternative battery technologies have been commercialized as discussed next.Dry cell
Dry cells do not have a fluid electrolyte. Instead, they use a moist electrolyte paste. Leclanché's cell is a good example of this, where the anode is a zinc container surrounded by a thin layer of manganese dioxide and a moist electrolyte paste of ammonium chloride and zinc chloride mixed with starch. The cell's cathode is represented by a carbon bar inserted on the cell's electrolyte, usually placed in the middle.Leclanché's simplified half reactions are shown below:
Mercury battery
This battery first appeared in the early 1940s. The mercury battery has many applications in medicine and electronics. The battery consists of a steel—made container in the shape of a cylinder acting as the cathode, where an amalgamated anode of mercury and zinc is surrounded by a stronger alkaline electrolyte and a paste of zinc oxide and mercury(II) oxide.Mercury battery half reactions are shown below:
Lead-acid battery
The lead-acid battery used in automobiles, consists of a series of six identical cells assembled in series. Each cell has a lead anode and a cathode made from lead dioxide packed in a metal plaque. Cathode and anode are submerged in a solution of sulfuric acid acting as the electrolyte.Lead-acid battery half cell reactions are shown below:

At standard conditions, each cell may produce a potential of 2 V, hence overall voltage produced is 12 V. Differing from mercury and zinc-carbon batteries, lead-acid batteries are rechargeable. If an external voltage is supplied to the battery it will produce an electrolysis of the products in the overall reaction (discharge), thus recovering initial components which made the battery work.
Lithium rechargeable battery
Instead of an aqueous electrolyte or a moist electrolyte paste, a solid state battery operates using a solid electrolyte. Lithium polymer batteries are an example of this; a graphite bar acts as the anode, a bar of lithium cobaltate acts as the cathode, and a polymer, swollen with a lithium salt, allows the passage of ions and serves as the electrolyte. In this cell, the carbon in the anode can reversibly form a lithium-carbon alloy. Upon discharging, lithium ions spontaneously leave the lithium cobaltate cathode and travel through the polymer and into the carbon anode forming the alloy. This flow of positive lithium ions is the electrical current that the battery provides. By charging the cell, the lithium dealloys and travels back into the cathode. The advantage of this kind of battery is that Lithium possess the highest negative value of standard reduction potential. It is also a light metal and therefore less mass is required to generate 1 mole of electrons. Lithium ion battery technologies are widely used in portable electronic devices because they have high energy storage density and are rechargeable. These technologies show promise for future automotive applications, with new materials such as iron phosphates and lithium vanadates.Flow battery/ Redox flow battery
Most batteries have all of the electrolyte and electrodes within a single housing. A flow battery is unusual in that the majority of the electrolyte, including dissolved reactive species, is stored in separate tanks. The electrolytes are pumped through a reactor, which houses the electrodes, when the battery is charged or discharged.These types of batteries are typically used for large-scale energy storage (kWh - multi MWh). Of the several different types that have been developed, some are of current commercial interest, including the iron/chromium flow battery, vanadium redox battery and zinc-bromine flow battery.
Fuel cells
Main article: Fuel cell
Fossil fuels are used in power plants to supply electrical needs, however their conversion into electricity is an inefficient process. The most efficient electrical power plant may only convert about 40% of the original chemical energy into electricity when burned or processed.To enhance electrical production, scientists have developed fuel cells where combustion is replaced by electrochemical methods, similar to a battery but requiring continuous replenishment of the reactants consumed.
The most popular is the oxygen-hydrogen fuel cell, where two inert electrodes (porous electrodes of nickel and nickel oxide [disambiguation needed]) are placed in an electrolytic solution such as hot caustic potash, in both compartments (anode and cathode) gaseous hydrogen and oxygen are bubbled into solution.
Oxygen-hydrogen fuel cell reactions are shown bellow:
Corrosion
Corrosion is the term applied to metal rust caused by an electrochemical process. Most people are likely familiar with the corrosion of iron, in the form of reddish rust. Other examples include the black tarnish on silver, and red or green corrosion that may appear on copper and its alloys, such as brass. The cost of replacing metals lost to corrosion is in the multi-billions of dollars per year.Iron corrosion
For iron rust to occur the metal has to be in contact with oxygen and water, although chemical reactions for this process are relatively complex and not all of them are completely understood, it is believed the causes are the following:- Electron transferring (Reduction-Oxidation)
- One area on the surface of the metal acts as the anode, which is where the oxidation (corrosion) occurs. At the anode, the metal gives up electrons.
- Electrons are transferred from iron reducing oxygen in the atmosphere into water on the cathode, which is placed in another region of the metal.
- Global reaction for the process:
- Standard emf [disambiguation needed] for iron rusting:
- One area on the surface of the metal acts as the anode, which is where the oxidation (corrosion) occurs. At the anode, the metal gives up electrons.
 . The electric circuit works as passage of electrons and ions occurs, thus if an electrolyte is present it will facilitate oxidation, this explains why rusting is quicker on salt water.
. The electric circuit works as passage of electrons and ions occurs, thus if an electrolyte is present it will facilitate oxidation, this explains why rusting is quicker on salt water.Corrosion of common metals
Coinage metals, such as copper and silver, slowly corrode through use. A patina of green-blue copper carbonate forms on the surface of copper with exposure to the water and carbon dioxide in the air. Silver coins or cutlery that are exposed to high sulfur foods such as eggs or the low levels of sulfur species in the air develop a layer of black Silver sulfide.Gold and platinum are extremely difficult to oxidize under normal circumstances, and require exposure to a powerful chemical oxidizing agent such as aqua regia.
Some common metals oxidize extremely rapidly in air. Titanium and aluminium oxidize instantaneouly in contact with the oxygen in the air. These metals form an extremely thin layer of oxidized metal on the surface. This thin layer of oxide protects the underlying layers of the metal from the air preventing the entire metal from oxidizing. These metals are used in applications where corrosion resistance is important. Iron, in contrast, has an oxide that forms in air and water, called rust, that does not stop the further oxidation of the iron. Thus iron left exposed to air and water will continue to rust until all of the iron is oxidized.
Prevention of corrosion
Attempts to save a metal from becoming anodic are of two general types. Anodic regions dissolve and destroy the structural integrity of the metal.While it is almost impossible to prevent anode/cathode formation, if a non-conducting material covers the metal, contact with the electrolyte is not possible and corrosion will not occur.
Coating
Metals can be coated with paint or other less conductive metals (passivation). This prevents the metal surface from being exposed to electrolytes. Scratch [disambiguation needed]es exposing the metal substrate will result in corrosion. The region under the coating adjacent to the scratch acts as the anode of the reaction.Sacrificial anodes
A method commonly used to protect a structural metal is to attach a metal which is more anodic than the metal to be protected. This forces the structural metal to be cathodic, thus spared corrosion. It is called "sacrificial" because the anode dissolves and has to be replaced periodically.Zinc bars are attached at various locations on steel ship hulls to render the ship hull cathodic. The zinc bars are replaced periodically. Other metals, such as magnesium, would work very well but zinc is the least expensive useful metal.
To protect pipelines, an ingot of buried or exposed magnesium (or zinc) is buried beside the pipeline and is connected electrically to the pipe above ground. The pipeline is forced to be a cathode and is protected from being oxidized and rusting. The magnesium anode is sacrificed. At intervals new ingots are buried to replace those lost.
Electrolysis
Main article: Electrolysis
The spontaneous redox reactions of a conventional battery produce electricity through the different chemical potentials of the cathode and anode in the electrolyte. However, electrolysis requires an external source of electrical energy to induce a chemical reaction, and this process takes place in a compartment called an electrolytic cell.Electrolysis of molten sodium chloride
When molten, the salt sodium chloride can be electrolyzed to yield metallic sodium and gaseous chlorine. Industrially this process takes place in a special cell named Down's cell. The cell is connected to an electrical power supply, allowing electrons to migrate from the power supply to the electrolytic cell.Reactions that take place at Down's cell are the following:
The emf [disambiguation needed] for this process is approximately -4 V indicating a (very) non-spontaneous process. In order for this reaction to occur the power supply should provide at least a potential of 4 V. However, larger voltages must be used for this reaction to occur at a high rate.
Electrolysis of water
Main article: Electrolysis of water
Water can be converted to its component elemental gasses, H2 and O2 through the application of an external voltage. Water doesn't decompose into hydrogen and oxygen spontaneously as the Gibbs free energy for the process at standard conditions is about 474.4 kJ. The decomposition of water into hydrogen and oxygen can be performed in an electrolytic cell. In it, a pair of inert electrodes usually made of platinum immersed in water act as anode and cathode in the electrolytic process. The electrolysis starts with the application of an external voltage between the electrodes. This process will not occur except at extremely high voltages without an electrolyte such as sodium chloride or sulfuric acid (most used 0.1 M).Bubbles from the gases will be seen near both electrodes. The following half reactions describe the process mentioned above:
Electrolysis of aqueous solutions
Electrolysis in an aqueous is a similar process as mentioned in electrolysis of water. However, it is considered to be a complex process because the contents in solution have to be analyzed in half reactions, whether reduced or oxidized.lectrolysis of a solution of sodium chloride
The presence of water in a solution of sodium chloride must be examined in respect to its reduction and oxidation in both electrodes. Usually, water is electrolysed as mentioned in electrolysis of water yielding gaseous oxygen in the anode and gaseous hydrogen in the cathode. On the other hand, sodium chloride in water dissociates in Na+ and Cl- ions, cation, which is the positive ion, will be attracted to the cathode (-), thus reducing the sodium ion. The anion will then be attracted to the anode (+) oxidizing chloride ion.The following half reactions describes the process mentioned:
When comparing the reduction potentials in reactions 2 & 4, the reduction of chloride ion is favored. Thus, if the Cl- ion is favored for reduction, then the water reaction is favored for oxidation producing gaseous oxygen, however experiments show gaseous chlorine is produced and not oxygen.
Although the initial analysis is correct, there is another effect that can happen, known as the overvoltage effect. Additional voltage is sometimes required, beyond the voltage predicted by the
 . This may be due to kinetic rather than thermodynamic considerations. In fact, it has been proven that the activation energy for the chloride ion is very low, hence favorable in kinetic terms. In other words, although the voltage applied is thermodynamically sufficient to drive electrolysis, the rate is so slow that to make the process proceed in a reasonable time frame, the voltage of the external source has to be increased (hence, overvoltage).
. This may be due to kinetic rather than thermodynamic considerations. In fact, it has been proven that the activation energy for the chloride ion is very low, hence favorable in kinetic terms. In other words, although the voltage applied is thermodynamically sufficient to drive electrolysis, the rate is so slow that to make the process proceed in a reasonable time frame, the voltage of the external source has to be increased (hence, overvoltage).Finally, reaction 3 is favorable because it describes the proliferation of OH- ions thus letting a probable reduction of H+ ions less favorable an option.
The overall reaction for the process according to the analysis would be the following:
Quantitative electrolysis & Faraday's Laws
Quantitative aspects of electrolysis were originally developed by Michael Faraday in 1834. Faraday is also credited to have coined the terms electrolyte, electrolysis, among many others while he studied quantitative analysis of electrochemical reactions. Also he was an advocate of the law of conservation of energy.First law
Faraday concluded after several experiments on electrical current in non-spontaneous process, the mass of the products yielded on the electrodes was proportional to the value of current supplied to the cell, the length of time the current existed, and the molar mass of the substance analyzed.In other words, the amount of a substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity passed through the cell.
Below a simplified equation of Faraday's first law:
- m is the mass of the substance produced at the electrode (in grams),
- Q is the total electric charge that passed through the solution (in coulombs),
- n is the valence number of the substance as an ion in solution (electrons per ion),
- M is the molar mass of the substance (in grams per mole).
Second law
Faraday devised the laws of chemical electrodeposition of metals from solutions in 1857. He formulated the second law of electrolysis stating "the amounts of bodies which are equivalent to each other in their ordinary chemical action have equal quantities of electricity naturally associated with them." In other terms, the quantities of different elements deposited by a given amount of electricity are in the ratio of their chemical equivalent weights.An important aspect of the second law of electrolysis is electroplating which together with the first law of electrolysis, has a significant number of applications in the industry, as when used to protect metals to avoid corrosion.
Applications
There are various extremely important electrochemical processes in both nature and industry, like the coating of objects with metals or metal oxides through electrodeposition and the detection of alcohol in drunken drivers through the redox reaction of ethanol. The generation of chemical energy through photosynthesis is inherently an electrochemical process, as is production of metals like aluminum and titanium from their ores. Certain diabetes blood sugar meters measure the amount of glucose in the blood through its redox potential.The nervous impulses in neurons are based on electric current generated by the movement of sodium and potassium ions into and out of cells, and certain animals like eels can generate a powerful voltage from certain cells that can disable much larger animals.
Main Articles:
Electroplating
Faraday's law of electrolysis
Redox reaction
Corrosion
Electrochemical cell
Standard electrode potential

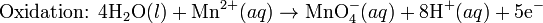


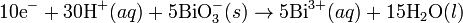
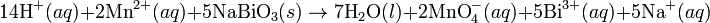



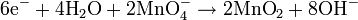











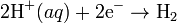
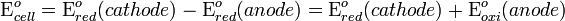

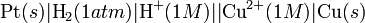
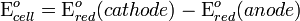








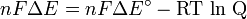







![E = E^{o}- {0.0592 V \over 2} log {[Cu^{2+}]_{diluted}\over [Cu^{2+}]_{concentrated}}\,](http://upload.wikimedia.org/math/e/6/8/e68834c6de33f75bd3dbfd8c5b18ac4c.png)